
|
|||
|---|---|---|---|
|
|
The effects of physical exercise on cancer: a review |
|
|
|
* PhD Student in Exercise Science: University of Northern Colorado (USA) ** Master of Science in Exercise Science: University of Northern Colorado (USA) *** Assistant Professor: Catholic University of Brasilia PhD in Exercise Science: University of New Mexico (USA) |
Claudio Battaglini * batt6879@unco.edu Becca Battaglini ** beccabattaglini@hotmail.com Martim Bottaro *** martim@ucb.br (Brasil) |
|
|
|
|
|||
|
|
http://www.efdeportes.com/ Revista Digital - Buenos Aires - Año 8 - N° 57 - Febrero de 2003 |
|
|
1 / 2
Definition of cancer
Cancer is new tissue growth resulting from the continuous and rapid production of abnormal cells that invade and destroy specific tissues. Cancer, which may arise from any type of cell in any body tissue, is not a single disease but includes a large number of diseases classified according to the tissue and type of cell in which new growth occurs (22). Several hundred classes of cancer exist, constituting (but not limited to) three major subtypes: carcinomas, sarcomas, and leukemia/ lymphomas. Carcinomas represent approximately 85% of all malignant tumors, sarcomas approximately 6%, and leukemia/ lymphomas account for about 5% of malignant tumors.
Cancer treatmentThere are three major cancer treatments available to patients: surgery, chemotherapy, and radiation. One approach in treating cancer is to remove all malignant cells surgery. In the past this meant the removal of all involved tissue and as much potentially involved tissue as possible, including adjacent tissues and lymph nodes. However for some tumors, notably cancer of the breast, radical surgery like mastectomy is not always necessary. Refinements in surgical techniques, improved knowledge of physiology, advances in anesthesiology, readily available blood products, and potent antibiotics have permitted less extensive surgery with more rapid recovery and less disability (7).
Radiation treatment is often used to destroy any cells that may be left behind after surgery. The use of radiation depends on the type of cancer and how far it has spread, and is usually recommended for patients where the cancer has spread outside the organ that was initially removed. Radiation is not always preceded by surgery, but can be given to a patient before any other treatment is administered.
On the electromagnetic spectrum, x-rays have a very high-energy and a very short wave length and they produce the ionization of atoms that occur when a beam of radiation passes through biological tissue (25). When radiation beams pass through living tissue, varying the characteristics of the radiation source can control the intensity, duration, and site of these ionization events. This control permits deliberate cellular destruction to occur. (25).
The physiochemical events that take place within the radiation-damaged cells are far from understood. Although there is little doubt that the important target site is the nuclear DNA, it is less common for the damage to be inflicted as a result of a ‘direct hit’. More commonly the effects are indirect, resulting in the production of unstable, highly reactive and short-lived free radicals, which in turn produce destruction of the normal DNA molecule with which they rapidly react (25). Fatigue, nausea, and skin burns are some usual side effects of radiation therapy.
Chemotherapy is the use of cytotoxic drugs in the treatment of cancer and is a systemic treatment, rather than a localized (surgery and radiation) treatment. Chemotherapy drugs are most active against frequently dividing cells (24). Normal cells with rapid growth changes that are most commonly affected by chemotherapy agents include bone marrow (platelets and red and white blood cells), hair follicles, the mucosal lining of the gastrointestinal (GI) tract, and skin and germinal cells (sperm and ova) (29).
Cytotoxic drugs, or chemotherapy, damage the reproductive integrity of cells. More rapidly growing tumors are more likely to respond to drug treatment. The explanation of why some types of cancer (lymphoma, testicular, and leukemia) are sensitive to cytotoxic drugs and other types (pancreatic and colon cancer) is certainly not just cell kinetics (24). This difference may also be related to mechanisms by which the cell death response is invoked, which may differ in efficiency or expression in one tumor compared with another. Some drugs (for example, methotrexate) are only effective at a particular phase of the cell cycle such as DNA synthesis, while others (alkylating agents) exhibit some action even against resting cells (4).
Chemotherapy, radiation, and sometimes surgery can cause damage to healthy tissues. Some of the long-range side effects of cancer treatment include cardiotoxicity, pulmonary toxicity, musculoskeletal alterations and endocrine system toxicity.
Cardiotoxicity is mainly found in patients receiving chemotherapy, especially with drugs that are widely used for treating breast, ovarian, lymphomas/ leukemia, and osteosarcomas (4). There are different modes of actual injury that can occur within the heart from chemotherapy treatment. One point of damage is to the cardiac myofibril. Within the myocyte, the sarcoplasmic reticulum swells and then damage to the cardiac myofibril results in degeneration of the mitochondria and nucleus, and loss of contractility of the myofibril. Another biochemical change is a decrease in cardiac gluthathione peroxidase (the enzyme that converts toxic substances into harmless ones) resulting in damage to the heart cell membrane by destructive free radicals (4). It is suggested that there is preferential binding of these free radicals to heart cell mitochondria, causing damage and possible alterations in calcium channel flow within the membrane. As more myofibrils are lost, the heart muscle has to work harder to compensate for the loss of pumping ability, and the heart enlarges resulting in increased oxygen demand. All cardiac damage, for the most part, is irreversible (5).
Radiation-induced cardiotoxicity is unlikely. However, there does seem to be a synergistic effect between chemotherapy and radiation (5). Many patients suffer from mild and often undetected cardiac toxicity. With cardiotoxicity, approximately 10% of the reported cases are fatal for both chemotherapy and radiation-induced.
The type of chemotherapy that is used for skin and lung cancer has been shown to contribute to pulmonary toxicity (4). In the lungs, there is a decrease in type I pneumocytes and a distribution and increase in type II pneumocytes into the alveolar space. Lasting chemotherapy effects may cause the alveolar septas to become thickened as well as decrease in number. Also, there is an increase in the amount of collagen secreted by the interstitial fibroblasts, resulting in generalized interstitial fibrosis of the lung (4). If damage continues, end-stage interstitial fibrosis reveals obliteration of the alveoli and dilated air spaces, followed by a thickened, stiff interstitium (4). Pulmonary dysfunction caused by chemotherapy is restrictive, with decreased lung volume, increased work of breathing, and impaired gas exchange.
Radiation effects on pulmonary function usually develop 2 to 3 months after treatment (5). Shielding the lungs during radiation treatment will help reduce the exposure, and decrease the incident of toxicity. Unfortunately this is an impractical solution. Shielding the lungs may increase the relapse of the patient since hidden tumor cells may be present in the shielded area (5).
Musculoskeletal alterations can occur with both chemotherapy and radiation. Uneven radiation to the vertebrae, soft tissue, and muscles (ex: radiation to one side of the body) for the treatment of intra-abdominal tumors frequently result in scoliosis, kyphosis or both (30). Late radiation damage to muscles can occur, especially following treatment of soft tissue sarcomas of the extremities. Mechanisms of injury have been identified (primarily from animal studies) that include a direct effect on myocytes resulting in cell death, vascular damage with ischemia, atrophy and fibrosis, and inflammation with preferential increase in type III collagen (24). Also the late effects of radiation on long bone include functional limitations, shortening of the extremity, osteonecrosis, increased susceptibility to fractures, and poor healing. Avascular necrosis and osteoporosis are also noted following radiation and prolonged use of corticosteroids (4).
The effects of chemotherapy on the musculoskeletal system are not as well documented. Lean body mass loss during cancer treatment is still not well explained. This reduction in skeletal muscle could be attributed to surgery reduction, treatment depletions, and inactivity during recovery.
Cancer treatment can adversely affect a number of endocrine functions, including metabolism, growth, secondary sexual development, and reproduction. These are later affects and result from damage to the target organ (thyroid, ovary, and testis) and the hypothalamic pituitary axis (30). Direct damage to the thyroid gland causes primary hypothyroidism, and decreased production of two hormones, thyroxine and triiodothyronine. These hormones have biological effects on oxygen consumption, the central and peripheral nervous system, skeletal and cardiac muscle, carbohydrate and cholesterol metabolism, and growth and development. Most findings of thyroid alterations have been due to radiation treatment (30).
Growth hormone deficiency due to cancer radiation treatment can affect many different biological functions. Aside from increasing linear growth, growth hormone is important in defining body composition, bone mineral density, muscle strength, exercise performance, cardiovascular system, metabolism, and immune function. Growth hormone deficient adults are at increased risk for osteoporitic fractures, heart disease, decreased lean body mass, and muscular strength (6, 8).
Secondary sexual development and reproduction are the last two areas in which the endocrine system can be affected by both chemotherapy and radiation. The age at the time of treatment, gender, total drug/radiation dose, and the use of alkylating agents (for chemotherapy treatment) are important risk factors in gonadal failure. In a large retrospective cohort study of 2283 survivors of childhood cancer, Bryne et al. (3) found that radiation therapy directed below the diaphragm depressed fertility in men and women by approximately 25%, and combination therapy involving radiation and alkylating agents reduced fertility to almost 50% of that in the control subjects (3). Chemotherapeutic agents that impair gonadal function (both men and women) may contribute to bone mineral density loss. In a study of 29 men previously treated for Hodgkin’s disease, a significant reduction in forearm cortical bone mineral content and lumbar spine bone mineral density was identified (30).
FatigueOne of the most prevalent side effects for 78%-96% of the cancer population is fatigue. Fatigue is characterized by the inability to perform physical tasks at one’s usual pace or strength, and by a slowing of the thinking processes that may involve failure of memory (19). The exact mechanism that causes fatigue in patients with cancer is not known. It is likely that many different mechanisms play a role.
A neurophysiological model, consisting of both central and peripheral components, has been proposed to study fatigue (22). The central component consists of the psyche/brain and spinal cord. The peripheral system consists of peripheral nerves, muscle sarcolemma, transverse tubular system, calcium release, actin/myosin interaction, cross-bridge tension and heat, and force/power output. Impairment of the central component causes lack of motivation, impaired spinal cord transmission, and exhaustion or malfunction of brain cells in the hypothalamic region. Damage to the peripheral component can cause impaired peripheral nerve function in transmission at the neuromuscular junction, thereby affecting fiber activation. Both types of damage may play a role in chronic fatigue (22).
Another perspective in the study of fatigue focuses on the reduction in skeletal muscle protein stores that may result from endogenous tumor necrosis factor (TNF), or from TNF administered as therapy. This muscle wasting would require individuals to exert an unusually high amount of energy to generate adequate contractile force during energy performance or during extended periods of sitting or standing (25).
The rapid reproduction of cancer cells can lead other cells to break down as well. When cells die or are destroyed, the cell releases potassium, uric acid, and phosphorous which are carried inside the cell (7). An increased amount of these substances can tax normal body function. These substances can cause the following to occur: the kidneys may have trouble removing water and waste from the body, muscles and nerves may be more excitable, the heart may not work as well, or may stop, and too much phosphorus can lead to a lack of calcium (7). This can lead to even more problems, because calcium has multiple effects on parathyroid function, kidney & other organs and tissues, modulation of enzyme function, intracellular & extracellular functioning, clotting factors and adhesion molecules.
Fevers due to cancer infections related to a weakened immune system can also cause the weak and tired feeling of fatigue. An increased body temperature not only makes cells work harder, but also leads them to consume more oxygen and nutrients to perform properly. If the cancer is in the bone marrow, the body is unable to make enough red blood cells to carry needed oxygen to the body’s cells. If the cancer is affecting other organs, the body may be unable to fully metabolize waste or absorb needed nutrients (30).
In patients with cancer, there are three major nutritional/ energy mechanisms that may be involved in fatigue: alteration in the body’s ability to process nutrients efficiently, increase in the body’s energy requirements, and decrease in intake of energy sources (see Table 1).
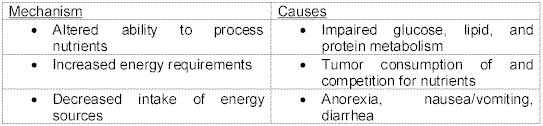
Table 1 - Nutritional/ Energy factors of fatigue. (National Cancer Institute)With all of the stated information above, there are many proposed factors that contribute to fatigue: neurophysiological model of central and peripheral components, the physical and psychological toll of the disease, the effects of chemotherapy, radiation or other medications, nutrition, and others such as fever.
Exercise benefits for cancer patientsOne of the hardest concepts to convince a cancer patient of is that exercise may help them to overcome the feeling of fatigue. Most patients would quickly disagree because they are too tired to do even normal daily activities. Exercise has been shown in many previous studies to play a more preventative role in the development of certain types of cancer (10,13,23,27). This concept is already known, but what about the benefits of exercise for a current cancer patient?
| Lecturas: Educación Física y Deportes · http://www.efdeportes.com · Año 8 · Nº 57 | sigue Ü |